Navigating definitions: Antimicrobials
Scientists use jargon and abbreviations all the time, and communication of science to a broad audience, which can also include scientists from diverse backgrounds and fields, can be jeopardized if such terms are not explained and defined in a simpler, accessible way. Even beginner scientists can struggle when reading scientific papers or navigating a new project. We often forget how difficult it was to understand concepts once we master them, which is commonly known as “the curse of knowledge”. Additionally, regulatory agencies also utilize a range of terms and definitions that are not always common across entities, and may be out of reach for the general population.
With that in mind, in this blog post I focus on explaining some important scientific concepts related to antimicrobial and resistance, which is a topic that affects directly or indirectly all of us and should be accessible to the general population, beginner scientists and researchers from other fields.
Antimicrobial
Antimicrobial, as defined by the Merriam-Webster dictionary, is a very broad term to describe a substance that kills or stops the growth of microorganisms. The root of the word indicates its definition: anti=against, micro=little and bios= life. The definition includes chemicals that act against all types of microorganisms: bacteria, viruses, fungi and protozoa, which can be either natural or synthetic (man-made) substances.
Antibiotic
Antibiotics, according to the FDA[1], are drugs meant to treat infections caused by bacteria. This definition is the most common one, but variations of it can be found in the literature, and they evolve and change with time. The term “antibiosis” was first utilized in 1889, and expressed the act of destroying life by one creature [2]. Probably because of the event of the discovery of penicillin (a natural substance produced by fungi) in 1928, by Alexander Fleming, the term antibiotics can often be seen associated exclusively to substances with natural origin. That removes from the definition a substantial portion of the drugs used to treat bacterial infections nowadays, which are chemically synthesized. In fact, in a scientific paper from the 50ths, the scientists defined antibiotics in an even more strict way, as being substances exclusively derived from fungi, excluding compounds that are also natural, but produced by plants [3].
Antiseptic
Antiseptics are products that control the growth of microorganisms, and are used topically (on the surface of the skin or living tissue), different from antibiotics which are used as medicine for humans and animals and act inside the body. The Food and Drug Administration (FDA) has several regulations regarding antiseptics. They divide antiseptics into two groups, health care and consumer antiseptics [4,5]. The over-the-counter antiseptics, available for the general population, are subsequently divided into two groups: washes and rubs. The first include antimicrobial soaps, and are meant to be rinsed off after use, while the second include hand sanitizers and antiseptics wipes, which are not to be rinsed off after use. The European Commission restricts even more the definition of antiseptics [6], including only products applied to non-intact skin and mucosa, while those applied to intact skin fall under their definition of disinfectants.
Biocide
The European Commission defines biocidal products as those “used to control unwanted organisms that are harmful to human or animal health or to the environment, or that cause damage to human activities” [6,7]. Even though the root of the word actually indicate a killing effect (bio=life, cide=that kills), the effect of the biocide will frequently depend on the concentration, and may result in halting growth but not necessarily killing. Biocides, according to their definition, include a range of products, such as insecticides, disinfectants and preservatives.
Disinfectant, sanitizer and chemical sterilant
Disinfectants are products meant to control the growth or kill undesired organisms from surfaces, devices and objects. It’s distinguished from antiseptics (used topically) and antibiotics (acting inside the body). Both the FDA and the U.S. Environmental Protection Agency (EPA) regulate disinfectants in the United States, while the Centers for Disease Control and Prevention (CDC) establishes rules regarding their correct use. The European Commission includes products meant to be used on intact skin under the definition of disinfectants.
Preservative
Preservatives are used in a range of products, such as food and cosmetics, to prevent microbial contamination. The European Commission provides regulation and guidance for the use of preservatives in Europe [8]. Preservatives are regulated in the US based on their use, by either the EPA or the FDA [9].
GRAS and GRAE
GRAS is the abbreviation for Generally Recognized As Safe. It usually applies to food additives, but is also used by the FDA for antiseptics. GRAE stands for Generally Recognized As Effective [5]. The first means that the product and its components have been demonstrated to be safe for a specific use, while the second means they have been shown to be effective, by doing what’s expected/promised to do. In the context of antimicrobial soaps, for example, manufacturers have to demonstrate that the benefits of their use overcome the risks. The data is gathered after laboratory experiments, and must show a satisfactory reduction in the number of microbes in the presence of the antiseptic product and its active ingredient.
Resistance and Tolerance
When an organism is not inactivated by in-use concentrations of a chemical, or a concentration that inactivates other strains of that organism, it is said to be resistant to that chemical [10]. For example, the emergence of carbapenem-resistant Klebsiella pneumoniae is a serious treat nowadays in hospital settings, since carbapenems (a type of antibiotic) are recommended to treat infections of such bacteria, and there are fewer treatment options when the bacteria acquire resistance to those antibiotics [11]. Other terms are also commonly used to describe this decrease in susceptibility, such as “insusceptibility”,“reduced susceptibility”, “tolerance”, and “tolerant” [10]. The terms “tolerance” and “resistance” may or may not be used interchangeably. In a scientific report for the Science Journal [12], researches have distinguished both definitions, using the term “tolerant” to describe those organisms that can temporarily avoid being killed by specific drugs by slowing their growth. There are standards used to determine resistance, and classify microorganisms into clinically susceptible, intermediate or resistant to a drug. In the United States, the Clinical and Laboratory Standards Institute (CLSI) provides guidelines and, in Europe, this task is the responsibility of the European Committee on Antimicrobial Susceptibility Testing (EUCAST) [13].
Cross-resistance
The term cross-resistance describes a phenomenon in which resistance or tolerance to a chemical or drug is developed after exposure to a different, but often related, chemical or drug. The term can be used, for example, to refer to a tumor that has been treated with one drug which may cause changes that reduce the efficacy of other drugs. Another example are bacterial cells that, after exposure to one antibiotic, became resistant to another antibiotic or even multiple other drugs [14].
Intrinsic and Acquired resistance
Resistance is usually divided into intrinsic and acquired, depending on whether its naturally observed in the microorganism, in the first case, or it was a result of changes rendering it resistant, in the second. Acquired resistance can occur through different paths. When mutations in the DNA occur and are selected for, being transmitted to daughter cells, it’s called vertical evolution. Another possibility is horizontal evolution, when antibiotic resistance genes are transmitted from one bacteria to another, which is the mechanism responsible for the majority of resistance problems [15].
MIC
The term MIC stands for Minimal Inhibitory Concentration and is determined for isolates of microorganisms. It represents the lowest drug concentration needed to prevent the visible growth of the microorganism, under defined test conditions. Inhibition does not necessarily means killing: the microbes can still be alive, even though growth cannot be observed. Growth of microbes can be visually observed by the turbidity in liquid media in which they grow, or the formation of a loan on top of a solid agar plate. There are several ways of determining the MIC, and the Clinical Laboratory Standards Institute, CLSI [16], describes in detail some of them.
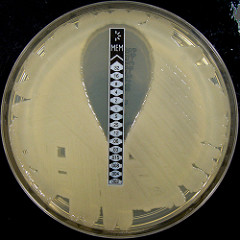
One method frequently used to determine the MIC of a bacteria is by using strips that are embedded with an antibiotic in a gradient of concentrations. The bacteria is spread in a solid media and the strip is placed on top of the plate. The antibiotic will diffuse (spread) around the strip. The plate is incubated (placed at a temperature optimal for the growth of the bacteria) and after some time, the growth of the bacteria can be observed around the areas where the antibiotic is present, as shown in the figure:
Credits: Nathan Reading
MIC50 and MIC90
The MIC50 and MIC90 are defined at the species level. They represent the lowest concentration of the antimicrobial at which 50 and 90% of the isolates for a specific species are inhibited, respectively. For example, in a study conducted in Turkey, researches obtained blood samples from 44 different patients that had the same disease, an infection caused by the bacteria Brucella melitensis, and determined the concentrations of several antibiotics that inhibited the growth of 50% (22 isolates) or 90% (40 isolates) of the bacteria [17]. Even though all isolates come from the same species of bacteria, differences in their MIC can occur, and may reflect the mechanisms of resistance acquired by specific isolates.
MBC
The Minimum Bactericidal Concentration or MBC can be determined by transferring microorganisms that were inhibited in MIC experiments into fresh media without the antimicrobial [10] and observing whether or not they are able to grow. The MBC represents the concentration of antimicrobial that actually kills, and not only inhibits, the microorganism.
Biofilm
Biofilms are structures composed of a hydrated matrix of polysaccharides and protein secreted by bacteria, and the bacteria itself [10]. The formation of this slimy layer by microorganisms poses a challenge for sanitization and treatment of infections. The collective grouping confers protection to the bacteria, and the concentrations of antimicrobials can be considerably lower inside the biofilm structure. Some examples of diseases commonly associated with biofilm formation are periodontitis and chronic lung infection in cystic fibrosis patients. Medical devices such as urinary catheters, prosthetic heart valves, and orthopaedic devices may also offer a surface for the attachment of microorganisms and the formation of biofilms [18].
Sources of information:
- Food and Drug Administration. Combating Antibiotic Resistance. <https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm092810.htm>
- Waksman SA. (1947). What Is an Antibiotic or an Antibiotic Substance? Mycologia V 39, No. 5 , pp. 565-569.
- Coatney GR, Greenberg J. (1952) The use of antibiotics in the treatment of malaria. Annals of the New York Academy of Sciences.
- Food and Drug Administration. Topical Antiseptic Products: Hand Sanitizers and Antibacterial Soaps. <https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm444681.htm>
- Food and Drug Administration. (2017) Safety and Effectiveness of Health Care Antiseptics; Topical Antimicrobial Drug Products for Over-the-Counter Human Use. 82 FR 60474, 21 CFR 310, 2017-27317.
- European Commission. Antibiotic Resistance Effects of Biocides. SCENIHR, 2009.<http://ec.europa.eu/health/scientific_committees/opinions_layman/en/biocides-antibiotic-resistance/l-3/2-main-uses-biocides.htm>
- European Commission. Biocides. <https://ec.europa.eu/health/biocides/overview_en>
- European Commission. Preservatives. http://ec.europa.eu/growth/sectors/cosmetics/products/preservatives_en
- Burt M.E. (1995) Regulation of preservatives in the USA. In: Morpeth F.F. (eds) Preservation of Surfactant Formulations. Springer, Dordrecht.
- Russell AD (2003) Biocide use and antibiotic resistance: the relevance of laboratory findings to clinical and environmental situations. The Lancet Infectious Diseases. V.3, p.794-803.
- Nordmann P, Cuzon G, Naas T. (2009) The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. The Lancet Infectious Diseases. V 9, I 4, P 228-236.
- Levin-Reisman et al. (2017) Antibiotic tolerance facilitates the evolution of resistance. Science 10.1126/science.aaj2191
- Wiegand I, Hilpert K, Hancock EW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols, V3 NO.2.
- ScienceDirect (Various authors). Cross-resistance. <https://www.sciencedirect.com/topics/immunology-and-microbiology/cross-resistance>
- Sommer MOA, Munck C, Toft-Kehler RV, Andersson DI. (2017)Prediction of antibiotic resistance: time for a new preclinical paradigm? Nature Reviews Microbiology v 15, p 689–696.
- CLSI. (2012) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition, Vol. 32 No. 2.
- Yamazhan T, Aydemir S, Tünger A, Serter D, Gökengin D. (2005) In vitro Activities of Various Antimicrobials against Brucella melitensis Strains in the Aegean Region in Turkey. Med Princ Pract;14:413–416.
- Stewart PS, Costerton JW. (2001) Antibiotic resistance of bacteria in biofilms.The Lancet, V 358.
Image by: Nathan Reading (https://www.flickr.com/photos/nathanreading/6652903223)